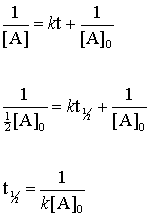half life formula for zero order reaction
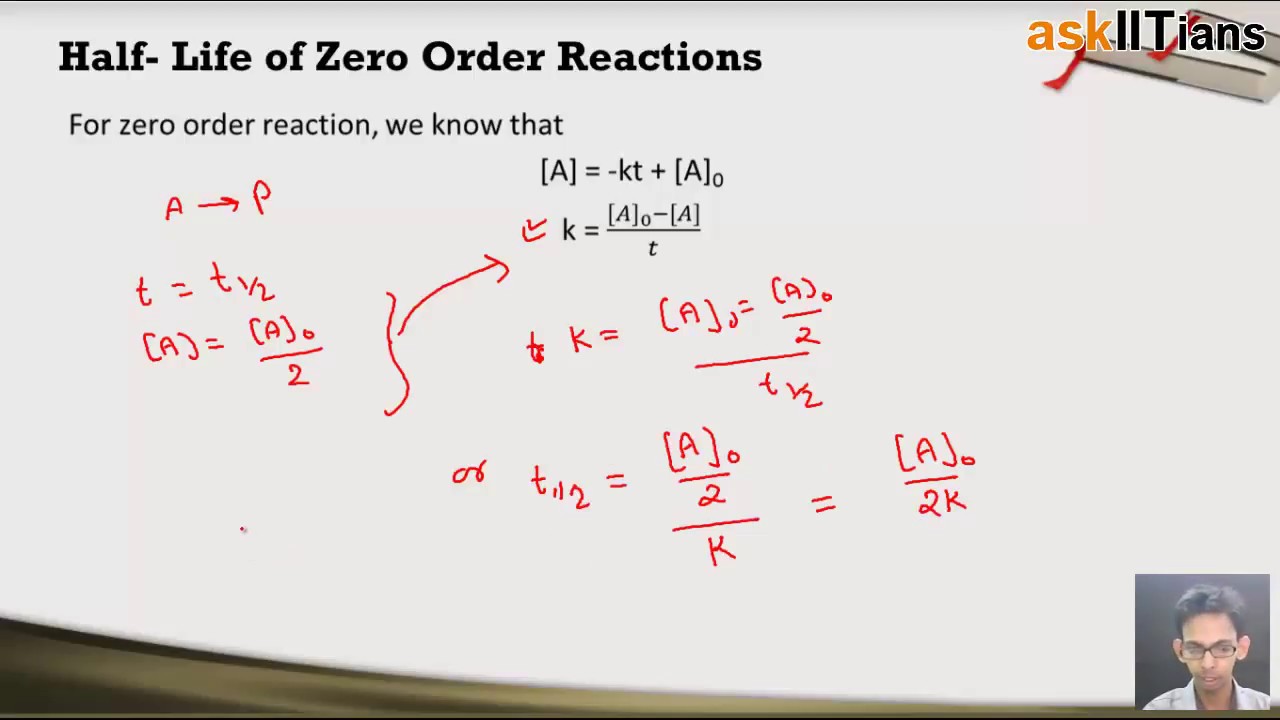
The timescale in which there is a 50 reduction in the initial population is referred to as half-life. Frac 1 A_02 frac 1 A_0 kt_ 12 frac 1 A_02 - frac 1 A_0 kt_ 12.

Half Life Of A Third 3rd Order Reaction Youtube
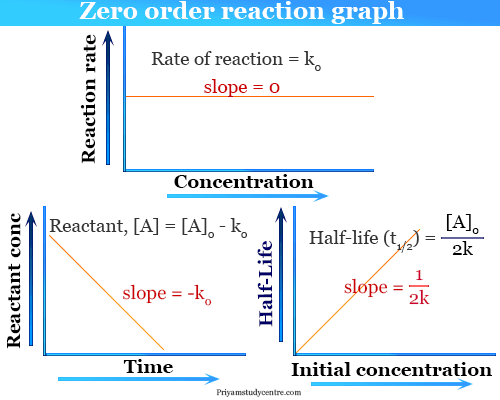
T 12 R 02k From the above relation we can say the Half-Life of a zero-order reaction is directly proportional to the initial concentration of the reactants and inversely.
. Zero-order reactions are typically found when a material that is. Converting a half life to a rate constant. 5 rows Zero-Order Reactions.
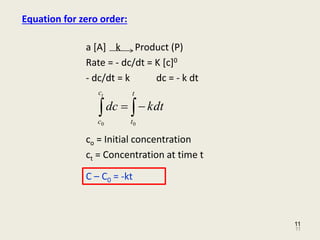
For a first-order reaction the. Half life in zero order reaction. The integrated rate law for the zero-order reaction A products is A_t -kt A_0.
Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions. The integrated rate law in the zero-order kinetics uses to derive half-life equations in chemistry.
This class of study uses to derive the half-life equation formulas in chemical kinetics reaction. The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure. For a zero-order reaction the half-life is given by.
For a zero-order reaction the mathematical expression that can be employed to. Equations for Half Lives. Determine the half-life of a zero order react.
T ½ A o 2k For a. For a zero order reaction A products rate k. TRA3C LO TRA3C5 EK Transcript.
Half life means 50 percent of reactants disappear in that time interval. Given below is the. Remember the half-life of a reaction changes with the order of the reaction.
It is to be noted that the half-life of a zero-order reaction is determined by the initial concentration and rate constant. Question - Half Life equation for zero order reaction is Answer - t12a2k. Half-Life of a Zero Order Reaction.
When t t ½ that is the half-life of the reaction completed the concentration of the. If we know the integrated rate laws we can determine the half. Substituting these terms into the rearranged integrated rate law and simplifying yields the equation for half-life.
As for other reaction orders an equation for zero-order. For a zero-order reaction increasing the concentration of the reacting species will not speed up the rate of the reaction. Latext_frac12 fracA_02klatex A 0 represents the initial concentration and k is the zero-order rate constant.
It is important to note that the formula for the half-life of a reaction varies with the order of the reaction. Determining a half life. The half-life of the reaction is denoted by t 12 and is expressed in seconds.
Now we have the following equation and can solve for eqt_ 12 eq. The rate constant for a Zero-order reaction rate of constant. The half-life is the time required for a quantity to fall to half its initial value as measured at the beginning of the time period.
Graphical relations and half lives. T12 A 02K. For the first-order reaction the half-life is defined as t 12 0693k.
Half-life is denoted by the symbol t 12. Click for more questions. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k.
The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction.

The Unit Of Rate Constant For Zero Order Reaction Is

Integrated Rate Laws Chemistry For Majors

Half Life Expressions Chemistnate
Kinetics Order Of Reactions Ppt Video Online Download

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com

What Is Half Life Period Derive Its Derivation

Which Of The Following Statements Are Corrects

Zero Order Reactions Video Kinetics Khan Academy
Zero Order Reaction Definition Examples Formula
Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube

Half Life Expressions Chemistnate
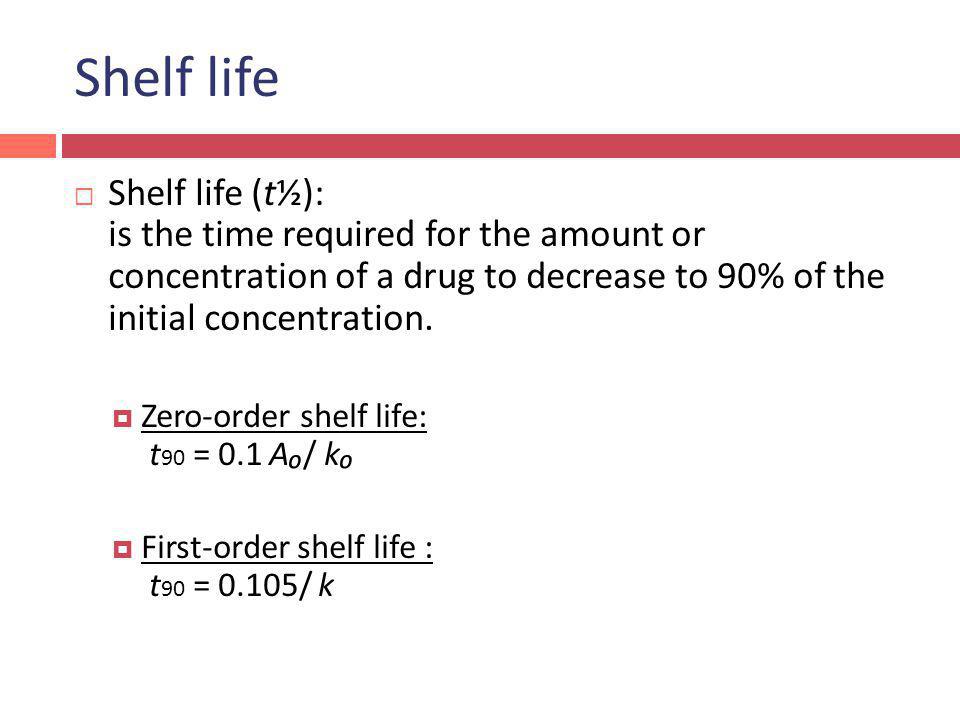
Kinetics And Drug Stability Ed
A Derive The General Form Of The Expression For The Half Life Of A First Order Reaction Sarthaks Econnect Largest Online Education Community